The microbiome hypothesis: Lipkin’s collaborative, part 1

A gut reaction is the problem in ME/CFS – that’s the main idea being pursued by Dr W. Ian Lipkin of the Center for Infection and Immunity at Columbia University. He believes that the body’s response to changes in the gut could be what’s driving ME/CFS for at least some patients.
Lipkin’s collaborative group, the Center for Solutions for ME/CFS, will test this theory as part of a $9.6 million, five-year research programme, which Lipkin was good enough to discuss with me via phone and email.
This huge research programme, which is funded by the National Institutes of Health (NIH), is made up of three main projects. This blog looks at the first two, which will use high-tech approaches to see if changes in the gut are causing changes in the body, particularly in the immune system. The third project, which looks at the biological response to exertion in ME/CFS, will be covered in the next blog.
Evidence from research supports the idea that gut problems could lead to ill health. Inside the gut, trillions of microbes – viruses, bacteria and fungi – form an ecosystem. Some are hitching a free ride, but many are useful, crowding out harmful microbes, helping us to digest our food or providing essential nutrients such as vitamin K.
A short video introduction to the microbiome from NPR
But sometimes the ecosystem can get out of balance, with too many of particular types of microbes or too few. This “dysbiosis” can play an important role in conditions such as inflammatory bowel disease.
This is where Lipkin comes in. He suspects dysbiosis as a possible cause for ME/CFS and sees two main possible mechanisms leading to disease. One could be through the metabolites produced by an unhealthy microbial ecosystem. Metabolites are small molecules that are a core part of the chemical processes that sustain life, and undesirable ones from microbes could enter the bloodstream from the gut.
Lipkin told me that these metabolites could be creating ME/CFS symptoms by, for instance, altering mitochondrial activity and so causing fatigue, or even affecting the brain, which could lead to brain fog and other cognitive problems.
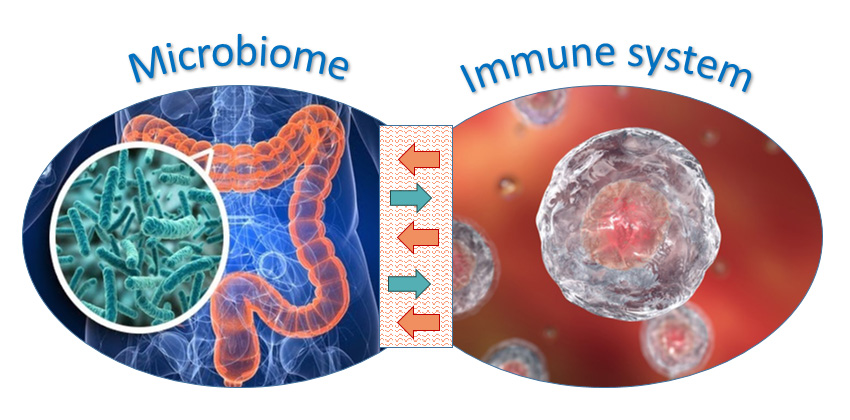
The microbiome and the immune system: it’s complicated
A second way that Lipkin believes that dysbiosis could cause ME/CFS is via the immune system. Recent research on microbial numbers indicates that there are roughly as many microbes in the gut as there are cells in the human body. That represents a serious threat of infection and requires a delicate balancing act for the immune system, dealing with dangerous pathogens without starting an inflammatory war. A fired-up immune system can lead to both fatigue and cognitive problems in many illnesses.
However, it’s a two-way relationship between the immune system and the microbiome. For instance, some gut bacteria produce the metabolite butyrate that stimulates certain types of T cells, helping to maintain the peace.

So there are at least two possible ways that the microbiome could be driving ME/CFS in at least some patients: microbiome metabolites that enter the blood could directly cause fatigue and cognitive problems; or more complex interactions between the microbiome and the immune system could lead to similar problems. But is either of these possibilities actually happening?
Dr Ian Lipkin is a good scientist to have on your case. He has probably discovered more viruses than anyone else. The Chinese government hired him to deal with the 2003 SARS outbreak. And he’s pioneered new technology throughout his career, most recently with a new way of detecting just about any human virus in a patient using a small chip. Versions of that technology will be used in this study.
Project 1: Mapping the microbiome
Lipkin has been working on the gut microbiome in ME/CFS for some time (see this recent paper, for example). Now, in this first collaborative project, he and his colleagues aim to find out if some or all ME/CFS patients have dysbiosis in the gut, by comparing patients with healthy controls.

All the microbes of the microbial ecosystem are technically known as the microbiota, but are usually referred to as simply the microbiome – the collection of all the genes that make up all the organisms. Surprisingly, it’s easier to find out the microbial make-up of the gut by analysing this huge set of genes – the microbiome – rather than by studying individual organisms.
Lipkin and colleagues will take this approach, using DNA sequencing and analysis of the microbiome to create microbial maps for all 107 patients and 97 healthy controls in the study.
The team will use a range of high-tech approaches to map the microbiome in a more sophisticated way than has been done before for ME/CFS, to provide the most accurate data possible. The resulting microbial maps will reveal if some or all patients have an off-balance microbiome that could be causing their illness – and the research team are looking at the viruses and fungi in the microbiome, not just the bacteria.
In addition, the team’s deep-sequencing method will provide insights into what genes are present in the whole microbiome, and how those genes might then affect a patient’s biology.
Project 2: Total molecular mapping of the immune system
 The next – and critical – step in Lipkin’s research programme will be to link any changes in patients’ microbiomes observed in Project 1 to changes in their immune systems, and this is the goal of Project 2.
The next – and critical – step in Lipkin’s research programme will be to link any changes in patients’ microbiomes observed in Project 1 to changes in their immune systems, and this is the goal of Project 2.
Lipkin will be using “omics” technology – a big data approach – to probe the working of immune cells in many different ways.
Omics offers a huge advantage over more traditional approaches in which, for example, researchers would have looked at the level of a single protein in a sample, such as the level of haemoglobin in blood. But with a protein omics approach, called proteomics, researchers look at a huge number of proteins in the sample – here the sample is all the immune cells that make up the white blood cells.
This broad omics approach can reveal far more about what’s going on in the immune cells than just looking at one or a few proteins.
Lipkin’s team hope to find differences between the immune cell proteins in patients and those of healthy controls, and that could reveal what’s gone wrong in ME/CFS.
The real bite of this study is that it won’t just use proteomics; it’s also using epigenomics to probe gene regulation, transcriptomics to see which genes are active, and metabolomics to probe which cellular chemical reactions are taking place.
These four omics approaches cover almost all fundamental levels of a cell’s molecular biology, providing a detailed map of activity in immune cells. This should give researchers extraordinary insight into the problems in the biology of ME/CFS patients.
Most processes in a cell begin with genes, the cell’s instructions written into its DNA. Genomics – the first and best known omics – looks at these instructions. But this study will instead use epigenomics, which looks at a level of broad gene regulation carried out by epigenetic tags. These tags are added to either sections of DNA or their scaffolding proteins and switch sets of genes on or off. Differences in regulation of genes between patients and controls revealed by epigenomics work could give clues about where problems arise for patients. So epigenomics provides the first level of information, as shown in the figure below.

Genes themselves do nothing until their DNA is transcribed (copied) into a similar molecule called RNA in a process known as gene expression. All the RNA transcripts in the sample are known as the transcriptome, and most of it consists of messenger RNA (mRNA), which tells cells which proteins to make. Other types of RNA can play a number of roles in the cell. Previous ME/CFS studies have looked at gene expression by looking for a large number of prespecified mRNAs, but not all of them. Transcriptomics offers a more comprehensive approach to understanding which genes are active in a cell.
The third level of omics is proteomics, which looks at the proteins. Proteins are the body’s ‘doing’ molecules, such as antibodies, cell receptors and enzymes.
The final level is metabolomics, looking at metabolites, such as glucose and some amino acids. Metabolites are key molecules in the chemistry of life: for example, they make up the energy cycle in the mitochondria – where Professor Ron Davis and others have identified abnormalities in ME/CFS patients.
So a cell’s DNA gets regulated, in part, by epigenetic tags (looked at by epigenomics) and is transcribed by RNA (looked at by transcriptomics) to make proteins (looked at by proteomics). Then enzymes, which are a type of protein, catalyse reactions involving metabolites (looked at by metabolomics).
Using omics to look at these four different levels of immune cells’ molecular biology – genes, RNA, proteins and metabolites – hugely improves the odds of findings where the problem lies.
Lipkin told me that you can start by finding an issue with a metabolite and then “swim upstream” to see if that problem is driven by a change in proteins, in RNA or in gene regulation. Similarly, you can swim downstream if you find an issue with broad gene regulation – does it make a difference to RNA, proteins and/or metabolites?

To make this aspect of Project 2 happen, Lipkin has recruited an impressive roster of omics experts to his collaborative, namely Dr John Greally for the epigenomics and transcriptomics work, Dr Ben Garcia for the proteomics, and Dr Oliver Fiehn, who leads one of the largest metabolomics labs in the world, to analyse metabolites. All of them are relatively new to ME/CFS and will bring fresh perspectives.
Probing antibodies for signs of autoimmunity and infection
In addition to the microbiome and related immune work, Lipkin’s team will also use elegant new technology that he developed to look for potential autoimmune problems and past infections that might have triggered the illness.
The approach focuses on antibodies, which bind very specifically to just one antigen – antigens are fragments of proteins, such as part of a virus’s protein coat, that trigger an immune response in the body. The antibody is like a specific lock that binds to just one antigen key.

The new technology essentially offers up a huge bunch of antigen keys (actually fragments of antigens called epitopes) and detects which one fits. If the antigen key is a protein from a pathogen, such as a flu virus, that indicates that the body has fought off an infection, possibly one that triggered ME/CFS. On the other hand, if the key is part of a “self” protein, that could be a sign of an autoimmune disease. Researchers will focus on any differences between patients and healthy controls.
And there’s more. The team will use additional technology – based on Lipkin’s high-tech way of detecting just about any human virus – to check the gut, saliva and blood for fungal or bacterial infections.
* * *
With these three approaches – a high-tech dive into the microbiome, linking changes in the microbiome to changes in the immune system at all molecular levels, and a search for what patients’ antibodies respond to – Lipkin’s investigation of what’s going on in ME/CFS patients is impressive. He hopes that this combination of depth and breadth will help to unlock ME/CFS.
One final point: Lipkin is not alone in thinking that problems with the microbiome might be driving ME/CFS via the immune system. Dr Derya Unutmaz is pursuing the same idea with his own collaborative. The main difference between the two researchers’ approaches is that while Lipkin has a more molecular focus, Unutmaz is focusing more on the different cells of the immune system, such as T cells and their subtypes. [Update: Unutmaz and Lipkin have announced a collaboration – both will apply their techniques to the same patients to give the deepest possible profiling of immune cells.]
Part 2: The heart of ME/CFS? Lipkin’s Collaborative probes the impact of exertion
The blog also features Lipkin’s bullish views on the future for ME/CFS.
Image credits: Dr Lipkin, Center for Infection & Immunity; Microbiome, source unknown (please contact me if it is yours); Immune cells, © Can Stock Photo / Kateryna_Kon; Metabolite & protein molecules, also Can Stock; Dr Fiehn, UC Davis;
